PQA 03 - PQA 03 Gynecological Cancer, Pediatric Cancer, and Professional Development Poster Q&A
3520 - The Safety and Efficacy of Cadonilimab in the First-Line Treatment of Recurrent or Metastatic Cervical Cancer: A Retrospective, Real-World Study
Monday, September 30, 2024
8:00 AM - 9:00 AM ET
Location: Hall C
Screen: 3
Ge Jin, PhD
The Fourth Hospital of Hebei Medical University
Shijiazhuang, China
Presenter(s)
G. Jin1, and J. Wang2; 1The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China, 2Department of Radiation Oncology, the Fourth Hospital of Hebei Medical University, Shijiazhuang, China
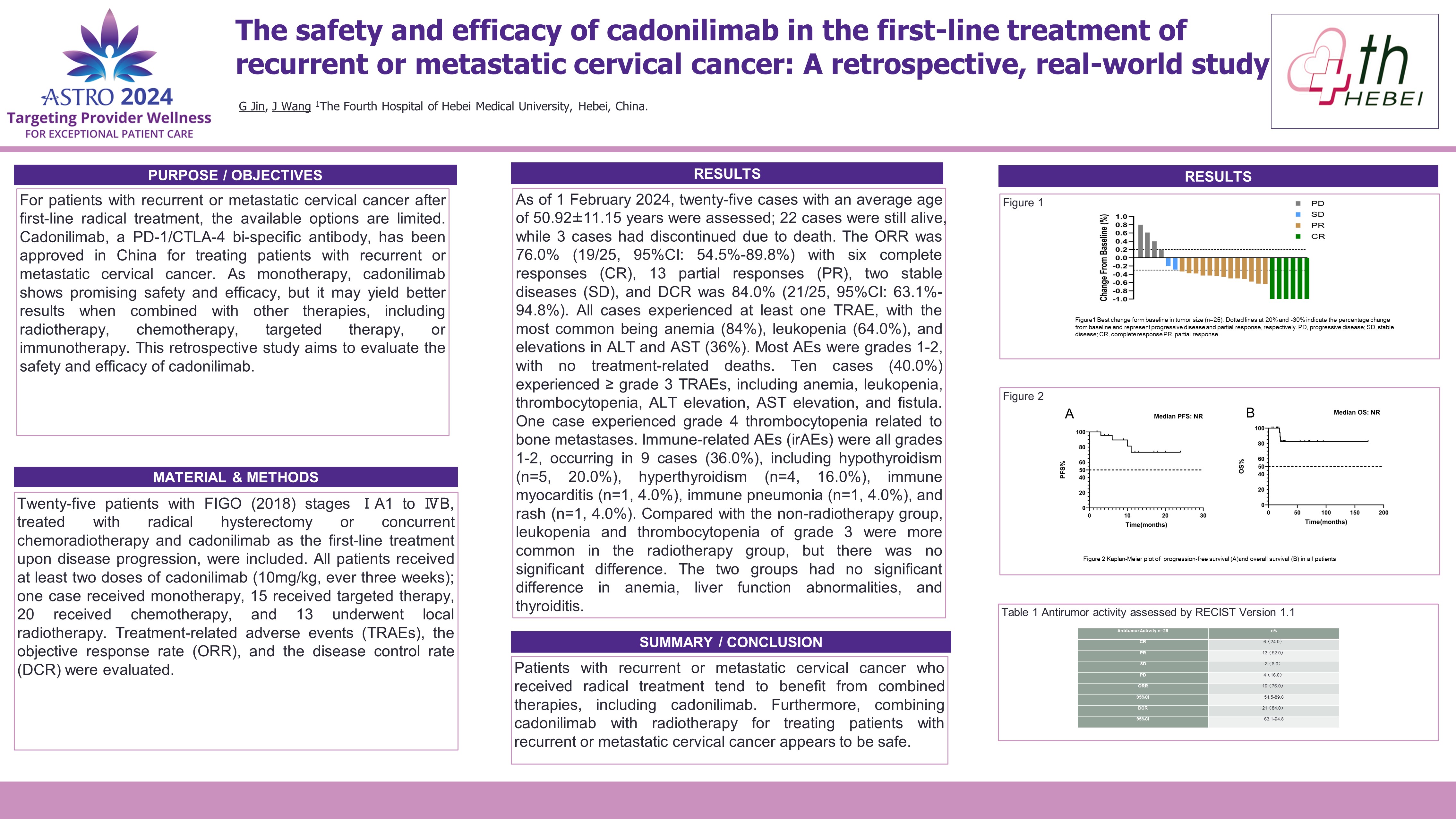
Purpose/Objective(s): For patients with recurrent or metastatic cervical cancer after first-line radical treatment, the available options are limited. Cadonilimab, a PD-1/CTLA-4 bi-specific antibody, has been approved in China for treating patients with recurrent or metastatic cervical cancer. As monotherapy, cadonilimab shows promising safety and efficacy, but it may yield better results when combined with other therapies, including radiotherapy, chemotherapy, targeted therapy, or immunotherapy. This retrospective study aims to evaluate the safety and efficacy of cadonilimab. Materials/
Methods: Twenty-five patients with FIGO (2018) stages IA1 to IVB, treated with radical hysterectomy or concurrent chemoradiotherapy and cadonilimab as the first-line treatment upon disease progression, were included. All patients received at least two doses of cadonilimab (10mg/kg, every three weeks); one case received monotherapy, 15 received targeted therapy, 20 received chemotherapy, and 13 underwent local radiotherapy. Treatment-related toxicities (TRAEs), the objective response rate (ORR), and the disease control rate (DCR) were evaluated.
Results: As of 1 February 2024, twenty-five cases with an average age of 50.92 ± 11.15 years were assessed; 22 cases were still alive, while 3 cases had discontinued due to death. The ORR was 76.0% (19/25, 95%CI: 54.5%-89.8%) with six complete responses (CR), 13 partial responses (PR), two stable diseases (SD), and the DCR was 84.0% (21/25, 95%CI: 63.1%-94.8%). All cases experienced at least one TRAE, with the most common being anemia (84.0%), leukopenia (64.0%), and elevations in ALT and AST (36.0%). Most AEs were grades 1-2, with no treatment-related deaths. Ten cases (40.0%) experienced =grade 3 TRAEs, including anemia, leukopenia, thrombocytopenia, ALT elevation, AST elevation, and fistula. One case experienced grade 4 thrombocytopenia related to bone metastases. Immune-related AEs (irAEs) were all grades 1-2, occurring in 9 cases (36.0%), including hypothyroidism (n=5, 20.0%), hyperthyroidism (n=4, 16.0%), immune myocarditis (n=1, 4.0%), immune pneumonia (n=1, 4.0%), and rash (n=1, 4.0%). Compared with the non-radiotherapy group, leukopenia and thrombocytopenia of grade 3 were more common in the radiotherapy group, but there was no significant difference. The two groups had no significant difference in anemia, liver function abnormalities, and thyroiditis.
Conclusion: Patients with recurrent or metastatic cervical cancer who received radical treatment tend to benefit from combined therapies, including cadonilimab. Furthermore, combining cadonilimab with radiotherapy for treating patients with recurrent or metastatic cervical cancer appears to be safe.
Purpose/Objective(s): For patients with recurrent or metastatic cervical cancer after first-line radical treatment, the available options are limited. Cadonilimab, a PD-1/CTLA-4 bi-specific antibody, has been approved in China for treating patients with recurrent or metastatic cervical cancer. As monotherapy, cadonilimab shows promising safety and efficacy, but it may yield better results when combined with other therapies, including radiotherapy, chemotherapy, targeted therapy, or immunotherapy. This retrospective study aims to evaluate the safety and efficacy of cadonilimab. Materials/
Methods: Twenty-five patients with FIGO (2018) stages IA1 to IVB, treated with radical hysterectomy or concurrent chemoradiotherapy and cadonilimab as the first-line treatment upon disease progression, were included. All patients received at least two doses of cadonilimab (10mg/kg, every three weeks); one case received monotherapy, 15 received targeted therapy, 20 received chemotherapy, and 13 underwent local radiotherapy. Treatment-related toxicities (TRAEs), the objective response rate (ORR), and the disease control rate (DCR) were evaluated.
Results: As of 1 February 2024, twenty-five cases with an average age of 50.92 ± 11.15 years were assessed; 22 cases were still alive, while 3 cases had discontinued due to death. The ORR was 76.0% (19/25, 95%CI: 54.5%-89.8%) with six complete responses (CR), 13 partial responses (PR), two stable diseases (SD), and the DCR was 84.0% (21/25, 95%CI: 63.1%-94.8%). All cases experienced at least one TRAE, with the most common being anemia (84.0%), leukopenia (64.0%), and elevations in ALT and AST (36.0%). Most AEs were grades 1-2, with no treatment-related deaths. Ten cases (40.0%) experienced =grade 3 TRAEs, including anemia, leukopenia, thrombocytopenia, ALT elevation, AST elevation, and fistula. One case experienced grade 4 thrombocytopenia related to bone metastases. Immune-related AEs (irAEs) were all grades 1-2, occurring in 9 cases (36.0%), including hypothyroidism (n=5, 20.0%), hyperthyroidism (n=4, 16.0%), immune myocarditis (n=1, 4.0%), immune pneumonia (n=1, 4.0%), and rash (n=1, 4.0%). Compared with the non-radiotherapy group, leukopenia and thrombocytopenia of grade 3 were more common in the radiotherapy group, but there was no significant difference. The two groups had no significant difference in anemia, liver function abnormalities, and thyroiditis.
Conclusion: Patients with recurrent or metastatic cervical cancer who received radical treatment tend to benefit from combined therapies, including cadonilimab. Furthermore, combining cadonilimab with radiotherapy for treating patients with recurrent or metastatic cervical cancer appears to be safe.
